The COVID-19 Saliva-based Screening Market to Gloat Over Hybrid Care Model
COVID-19 Saliva-based Screening Market 2022-2031
New Study Reports “COVID-19 Saliva-based Screening Market 2022, Analysis, Growth, Size, Trends Forecasts 2031″ has been Added on PMR.
Report Overview
Starting with the overview of the COVID-19 Saliva-based Screening Market, it presents an overall analysis of the latest trends that are prevalent in the industry. The report begins with the description of the market surroundings and the analysis of the size and forecast of product or service based on regions and applications. In addition to it, the report also introduces the market competitive landscape among the vendors and manufacturers that contribute to the growth of the product or service. The company profiling and market price analysis in relation to the value chain features is also covered in the report. The data experts have examined and scrutinized the industry trends in the key regions. 2022 has been considered as the base year, with the forecast period extending to 2031.
As per Persistence Market Research’s latest industry analysis, the global COVID-19 saliva-based screening market was valued at over US$ 1.7 Bn in 2020, and is expected to exhibit a declining CAGR of -3% over the forecast period of 2022 to 2031.
Get Free Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/32712
COVID-19 saliva-based screening is attaining traction in the existing situation, as it is patient-friendly, easy to use, and has a shorter test-to-result timeline. COVID-19 saliva-based screening plays a dynamic role in screening asymptomatic persons, thus sustaining market growth. This is mainly owing to numerous advantages offered by saliva-based tests, growing product approvals by FDA, and a shift toward point-of-care testing.
With the onset of the COVID-19 pandemic, several key players have entered the screening market. Advertising and expansion strategies are also playing an important role in generating market revenue. Furthermore, manufacturers are investing extensively in research & development activities to develop novel techniques that can allow rapid testing and accurate results.
Product launches, approvals, and acquisitions & mergers are rampant in this market space:
- Abbott launched ID NOW, a molecular POCT for detecting novel coronavirus (COVID-19), authorized by the U.S. FDA-issued Emergency Use Authorization (EUA). It is small, lightweight (6.6 pounds) and portable (size of a small toaster), and uses molecular technology that delivers positive results in as little as five minutes and negative results in 13 min.
- In April 2021, Chembio Diagnostics, Inc. announced the launch of an FDA emergency use authorization-approved, in-licensed rapid POCT for COVID-19/Flu A&B test, for use in decentralized and traditional testing settings.
- Thermo Fisher Scientific acquired Mesa Biotech, a developer of hand-held test devices for COVID-19 detection. Mesa received approximately US$ 450 Mn in cash and became eligible for an additional US$ 100 Mn based on the completion of certain pre-discussed milestones. This acquisition enabled Thermo Fisher Scientific to accelerate the availability of reliable and accurate advanced molecular diagnostics at POCT.
Company Profiles:
- Thermo Fisher scientific
- Qiagen
- Hologic
- Takara Bio Inc
- Arcis Biotechnology Limited
- GeneProof
- Abacus ALS
- Chai Inc.
- Kolplast Group
- Lucence Health INc
- Vitagene
- Therma Bright Inc
- ACON Laboratories, Inc
- ALL PHARMA
- Norgen Biotek Corp.
- Assure Tech. (Hangzhou) Co., Ltd
- Beijing Hotgen Biotech Co., Ltd
- Nantong Diagnos Biotechnology Co., Ltd
- NeuMoDx Molecular, Inc.
- KYODO INTERNATIONAL INC.
- Canvax
- Zymo Research Corporation.
- Salimetrics
- NEST Scientific USA
- Miraclean Technology Co. Ltd
- Mawi DNA Technologies
- Cell Projects Ltd.
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/32712
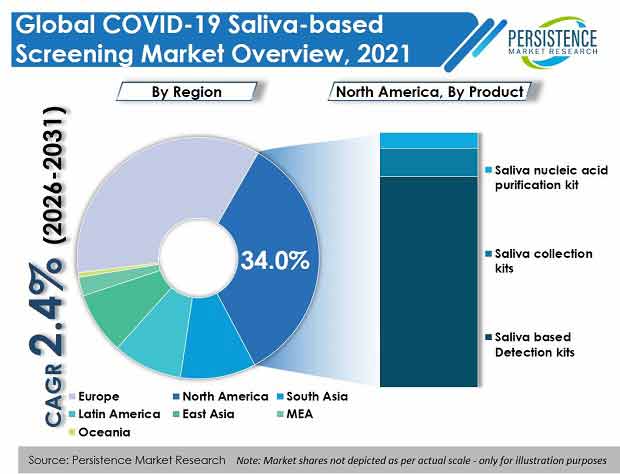
Key Takeaways from Market Study
- Based on product, saliva-based detection kits are leading with over 83% market share.
- By technology, RT-qPCR technology holds around 49% market value share, globally, primarily due to increasing focus on rapid testing and growing research activities.
- COVID-19 diagnostics is estimated to dominate the market by application. This segment accounted for approximately 63% share of the market, primarily due to rising prevalence of Covid-19 and increasing demand for COVID-19 diagnostics
- Diagnostic laboratories dominate the market among the end users with a market share of 27%.
- By region, Europe is set to dominate the global market with a value share of around 36%.
- North America is slated to be the second-largest leading market with a value share of 34% through 2031.
“Increasing adoption of COVID-19 saliva-based screening, rising ageing population susceptible to COVID-19, and continuous guidance from governments to support the response effect of the pandemic are estimated to boost market growth over the coming years,” says an analyst of Persistence Market Research.
Market Competition
New product approvals, launches, collaborations, agreements, and partnerships have emerged as the main growth strategy implemented by leading players. Acquisitions allow a company to expand its product portfolio. The company that is acquired possesses well-established diagnostic products and solutions, which become a good revenue source for the acquirer. This also enables the company to enter new emerging markets as well as existing markets.
- In April 2021, Vatic Health received the CE mark for a saliva antigen test as an on-the-spot test for the SARS-CoV-2 virus. The test has also completed the Medicines and Healthcare Products Regulatory Agency (MHRA) registration in the U.K.
What Does the Report Cover?
Persistence Market Research offers a unique perspective and actionable insights on the COVID-19 saliva-based screening market in its latest study, presenting a historical demand assessment and projections for 2020 – 2031.
The research study is based on the product (saliva collection kits, saliva nucleic acid purification kit and saliva-based detection kits), technology (direct sample to PCR, RT-qPCR and lateral flow assay), application (COVID-19 research and COVID-19 diagnostics), end user (diagnostic laboratories, hospitals & clinics, academic and research institutes, biopharma companies, long term care facilities, home care settings and others), across seven key regions of the world.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/32712
About Us:
Persistence Market Research is here to provide companies a one-stop solution with regards to bettering customer experience. It does engage in gathering appropriate feedback after getting through personalized customer interactions for adding value to customers’ experience by acting as the “missing” link between “customer relationships” and “business outcomes’. The best possible returns are assured therein.
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com
Website – https://www.persistencemarketresearch.com
Comments
Post a Comment